Management of Chronic Osteomyelitis and Antibiotic Stewardship; An Effort to Delay the Post-Antibiotic Era
By Abeera Ahmed1, Ahmed Mushtaq2, Irfan Ali Mirza3, Fatima Sana1, Syed Rehan Asghar Naqvi4Affiliations
doi: 10.29271/jcpsp.2024.04.474ABSTRACT
Objective: To develop an effective antimicrobial strategy for the management of chronic osteomyelitis.
Study Design: Observational study.
Place and Duration of the Study: Departments of Microbiology and Orthopaedics, Combined Military Hospital Malir, Karachi, Pakistan, from January 2021 to February 2022.
Methodology: Bone biopsies of 45 enrolled participants were taken for microbiological evaluation. Intravenous antibiotic therapy was begun as per empirical therapy based on the local antibiogram and antibiotic policy. Once the susceptibility pattern was available, targeted therapy started and continued for 28 to 42 days. Patients were evaluated based on inflammatory markers and clinical conditions for a minimum of six months to a maximum of one year.
Results: Out of the 45 patients, the majority 29% were soldiers, 40% belonging to the age group of 31-60 years. The common predisposing factor was trauma/fractures followed by diabetes and implants leading to chronic sinus discharge and decubitus ulcers. The most commonly isolated organism was Staphylococcus aureus (38%) followed by Methicillin-resistant Staphylococcus aureus (MRSA) (31%). Cotrimoxazole and Rifampicin turned out to be good treatment options. Only 4.4% showed unsatisfactory prognosis, nonetheless, no mortality was observed during the course of treatment.
Conclusion: In this study, highly resistant strains were observed with limited treatment options for chronic osteomyelitis, however, effective stewardship programmes with accurate diagnostic reporting and judicious use of antimicrobials can prevent overuse of the valuable resources.
Key Words: Antimicrobial stewardship, Osteomyelitis, Methicillin-resistant Staphylococcus aureus, Empirical therapy, Antimicrobial resistance.
INTRODUCTION
Osteomyelitis (OM), a term used for bone infection, is as old as civilisation and continues to be a major issue in the present era of medicine owing to its high morbidity and serious consequences.1,2 A relapsing and persistent infection characterised by low-grade inflammation that progresses over months to years is known as chronic osteomyelitis (COM).3,4 Chronic osteomyelitis is much more common in underdeveloped countries, due to poor healthcare facilities and limited resources.5 To arrest COM, most experts consider it essential to provide adequate drainage and intravenous antibiotic treatment for at least 4 to 6 weeks.2,4 Proper selection of therapy should always be made on the basis of correct identification of the causative organism(s) and knowledge of the susceptibility of bacteria to antibiotics.5
It has been observed that long-lasting disease and severe disability are frequently seen in patients with poor nutritional status and inadequate healthcare provision.6,7 Bone biopsy and its culture is gold standard for the diagnosis of OM in order to ascertain the causative organism,4,7 the foundation of effective management of OM is based on prompt diagnosis and aggressive treatment with thorough debridement and culture-directed antibiotic therapy.8,9 In 50 to 70% of OM cases, the most commonly isolated organism is Staphylococcus aureus (S.aureus). The rate of Methicillin-Resistant Staphylococcus aureus (MRSA) causing OM varies from 10 to 59% in different studies. Patients with history of recurrent debridement are frequently found to have Gram-negative and polymicrobial infections.10
Treatment for complicated Osteomyelitis typically invol-ves a short course of intravenous antibiotics, followed by a longer course of oral antibiotics. To achieve optimal results, antibiotics should be chosen according to local antibiotic sensitivity data (antibiogram).11,12 Research in this area can help guide clinicians in selecting appropriate antibiotic regimens and improve treatment outcomes. The objective of this study was to optimise the treatment strategy by evaluating different antimicrobial options for chronic osteomyelitis.
METHODOLOGY
This study was conducted in the Microbiology and Orthopaedics departments of Combined Military Hospital Malir, Karachi, Pakistan, from January 2021 to February 2022 after obtaining approval from the institutional research and ethical committee (file no: 24/2022/Trg/Ere). Hospitalised patients with a diagnosis of osteomyelitis based on clinical, radiological and/or microbiological methods, irrespective of their gender and socio-economic conditions, were screened for COM at the hospital. COM was defined as a bone and bone marrow infection that became worse or progressed and had not improved either clinically or microbiologically, even after one month of management. Patients who had given informed consent and were of age more than 13 years fulfilling the predefined criteria of COM were included in this study. Patients less than 13 years, not meeting the criteria of COM or refused to give informed consent and those who did not remain in follow-up for up to 1 year were excluded. The studied populace was assigned as specimen collection at least 48 hours after the stoppage of all antibiotic therapy, and bone biopsies (bone marrow, sequestra and cortical bone) collected surgically from the infected site for microbiological evaluation were only accepted, whereas the debrided tissues were rejected. Orthopaedic need was asked to specify further regarding the surgical entree to the infected bone, whether it was made through unbroken skin or breached skin area. Two specimens from the same site were collected, one in a brain heart infusion bottle (BHI) for aerobic culture and the second in Robertson cooked meat medium (RCM) for anaerobic culture. The specimens were transported, processed, and inoculated on different media as per routine and standard laboratory techniques, including special stains for bacteria, fungi, and mycobacteria for microscopic identification.
The phenotypic identifications based on biochemical reactions for gram-negative organisms were done by using either an analytical profile index (API 10 S, bio Merieux) or an automated system (VITEK2; bioMerieux) for both Gram-positive and Gram-negative isolates, and for anaerobes (Rapid ID32A, bioMerieux) as per standard protocols. Additional tests like the Coagulase test were performed and DNase plates were inoculated to further differentiate Staphylococcus aureus from Coagulase Negative Staphylococci. Modified Kirby Bauer disc diffusion method was used for susceptibility profile and in certain cases, minimum inhibitory concentrations (MICs) were checked by using an automated VITEK 2 system as per the Clinical and Laboratory Standards Institute (CLSI) guidelines 2021.13 Cefoxitin disc (FOX 30µg) was tested in Gram-positive isolates as a surrogate marker for Methicillin-resistant Staphylococcus aureus (MRSA) which means those isolates were intrinsically resistant to all available beta-lactam drugs including their combination antibiotics except 5th generation Cephalosporins which have Anti-MRSA activity. Intravenous (IV) antibiotic therapy was begun as per empirical therapy based on the local antibiotic policy, already defined before the start of the study (Table I). Once the susceptibility pattern of the bone-infecting organisms was established, targeted therapy started and continued for 28 to 42 days (Table I). To evaluate the success of therapy, available patients were observed by the researchers at the outpatient unit for a minimum of 6 months and up to a year.
Patient data were entered in the Microsoft Excel sheet 2013 by using the non-probability consecutive sampling technique. This included the age, gender, occupation, hospitalisation time, bone involved, mechanism of bone infection, the clinical condition of the skin incised by the surgeon to take the bone specimen, any comorbidities, inflammatory marker, c-reactive protein (CRP) to assess prognosis, microbiologic identification and susceptibility pattern of organisms grown aerobically and anaerobically, empirical and definitive therapy and its response. This data was then analysed by Statistical Package for the Social Sciences (SPSS) version 25. Frequency and percentages were calculated both for qualitative (such as demographic and clinical details etc.) and quantitative variables (such as age).
RESULTS
A total of 45 confirmed cases of COM based on their clinical, radiological, and positive bone culture results were admitted to the hospital for further management. Out of these 45 patients, 24 (53%) were males and 21 (47%) were females, reflecting male predominance belonging to the age group 31-60 years, 18 (40%) with mean age 46±19 years. Fifteen (33%) patients were in 61-80 years age group while 12 (27%) were in 14-30 age group.
The majority of the patients 16 (35.5%) were serving soldiers followed by 10 (22%) housewives, retired soldiers 7 (16%), students 6 (13%), and farmers 6 (13%). Most of the patients almost 17 (37.8%) were already known cases of COM and presented with acute exacerbation either due to prolonged usage of steroids or other associated comorbidities, such as diabetes mellitus, rheumatoid arthritis, implant, and trauma/fractures. However, 28 (62.2%) had no comorbid conditions.
Unhealed, infected wounds with sinus formation and chronic discharge 12 (27%) from the site of old fractures or recent un-united right femur 19 (42%)/or left tibia 16 (36%) fractures were predominately found. Fistula formation 8 (18%) or sinus formation was also observed in the case of total knee replacement (TKR), out of which 4 (9%) had prosthesis or implant infection.
The majority of the patients responded well to the treatment with 95.5% recovering fully after 8 weeks of treatment except for two patients, with TKR showed treatment failure and were treated for an extended period, one case was due to candida which was treated till 6 months for complete remission and other was an elderly patient infected with MRSA who got treatment for another 4 weeks. Both of these patients had a knee replacement history.
Table I: Management plan of 45 patients hospitalised with COM.
|
Isolated Organisms |
Empirical therapy |
Targeted therapy after culture report |
|
Gram-Positive Organisms |
I/V Co-amoxiclav (8 hourly) + Amikacin |
I/V Vancomycin X 02 weeks in case of MRSA otherwise continued with Inj Co-amoxiclav than de-escalated to Oral Cotrimoxazole + Rifampicin (06 weeks) |
|
Gram-Negative Organisms |
I/V Meropenem (8 hourly) + Linezolid |
I/V Meropenem X 02 weeks in case of XDR isolates otherwise continued with the same regimen and than de-escalated to Oral Cotrimoxazole + Minocycline (06 weeks) |
|
Anaerobe |
I/V Meropenem (8 hourly) + Linezolid |
I/V Meropenem x 02weeks Oral Co-moxiclav + metronidazole (06weeks) |
|
Candida albicans |
I/V Augmentin (8 hourly) + Amikacin |
I/V Fluconazole X 02 weeks Oral Fluconazole x 06months |
Table II: Antimicrobial resistance pattern of isolated organisms from bone culture.
|
Antibiotics/ Antifungals |
Gram Positive Organisms (31) |
Gram Negative Organisms (11) |
Fungi (2) |
||
|
Staphylococcus aureus (17) |
MRSA (14) |
Serratia marcescens (9) |
Proteus mirabilis (2) |
Candida albicans (2) |
|
|
Ampicillin |
100% |
100% |
IR* |
100% |
Not tested |
|
Augmentin* |
0% |
100% |
IR |
100% |
|
|
Amikacin |
Not reported |
Not reported |
45% |
47% |
|
|
Cloxacillin* |
0% |
100% |
IR |
NT |
|
|
Clindamycin |
22% |
25% |
NT |
NT |
|
|
Ceftriaxone* |
NT* |
NT |
96% |
98% |
|
|
Ciprofloxacin |
71.4% |
88% |
91% |
0% |
|
|
Co-trimoxazole |
3% |
6% |
0% |
3% |
|
|
Doxicycline |
2% |
5.8% |
9% |
IR |
|
|
Fluconazole |
NT |
NT |
NT |
NT |
0% |
|
Levofloxacin |
70.6% |
86% |
89% |
0% |
Not tested |
|
Linezolid |
0% |
0% |
NT |
NT |
|
|
Minocycline |
0% |
2% |
0% |
IR |
|
|
Imipenem |
NT |
NT |
0% |
0% |
|
|
Meropenem |
NT |
NT |
0% |
0% |
|
|
Penicillin |
100% |
100% |
NT |
NT |
|
|
Piperacillin-tazobactum |
NT |
NT |
95% |
97% |
|
|
Polymyxin |
NT |
NT |
IR |
IR |
|
|
Rifampicin |
0% |
0% |
NT |
NT |
|
|
Vancomycin |
0% |
0% |
NT |
NT |
|
Initially, after taking bone culture by bone biopsy specimen, patients were placed on pre-defined empirical therapy based on the latest formulated antibiogram from the orthopaedic department according to which Amikacin plus Co-amoxiclav was suggested as empirical therapy but after the culture reports the targeted therapy was started as per culture and sensitivity results and further antibiotics were escalated as the clinical condition of the patient as shown in Table II. In the studied populace, the most commonly isolated organisms include 31 (69%) gram-positive cocci with 17 (38%) Staphylococcus aureus, 14 (31%) Methicillin-resistant Staphylococcus aureus followed by 11 (24%) gram-negative organisms including 9 (20%) Serratia marcescens and 2 (4%) Proteus mirabilis. In 1 (2%) case, initially no growth had seen on routine conventional media but when the author dealt with it again and applied it as anaerobic culture then appreciated the growth of an anaerobe which was gram-positive cocci on gram stain, further identification was not carried out due to lack of availability of anaerobic identification test. Two cases 2 (4%) of invasive yeast infections were also identified, one with history of prosthesis implant and the other of the chronic discharging sinus in which isolated yeast was identified as Candida albicans as mentioned in Figure 1. All the gram-positive cocci were found 100% susceptible to linezolid, vancomycin, and rifampicin, keeping in view this susceptibility pattern the authors treated MRSA-positive cases with intravenous vancomycin 12 hourly for 2 weeks followed by oral drugs Cotrimoxazole and Rifampicin for 6 weeks. De-escalation of drugs based on patient response monitored with CRP as mentioned in Figure 1. In Gram-negative isolates, Carbapenems, Doxycycline and Minocycline were susceptible in most of the cases. For patients in which isolated organisms were Gram-negative rods, Meropenem was given 08 hourly for 2 weeks followed by oral cotrimoxazole and Minocycline for 04 weeks. Gram-positive and Gram-negative isolates were found susceptible to Cotrimoxazole with 100% susceptibility. Anaerobic bone infection was treated with I/V Meropenem x 8 hourly for 02 weeks followed by Clindamycin and Metronidazole orally for 6 weeks. Patients infected with Candida albicans were placed on I/V Fluconazole for 2 weeks followed by oral Fluconazole for 6 months (Table I). Patients were followed for a period of 1 year and their monitoring was done based on the falling pattern of CRP to normal after 12 months with the improved clinical status of the patient (Figure 1). Complete remission was noted in 43 (96%) patients while 2 (4%) patients showed poor prognosis.
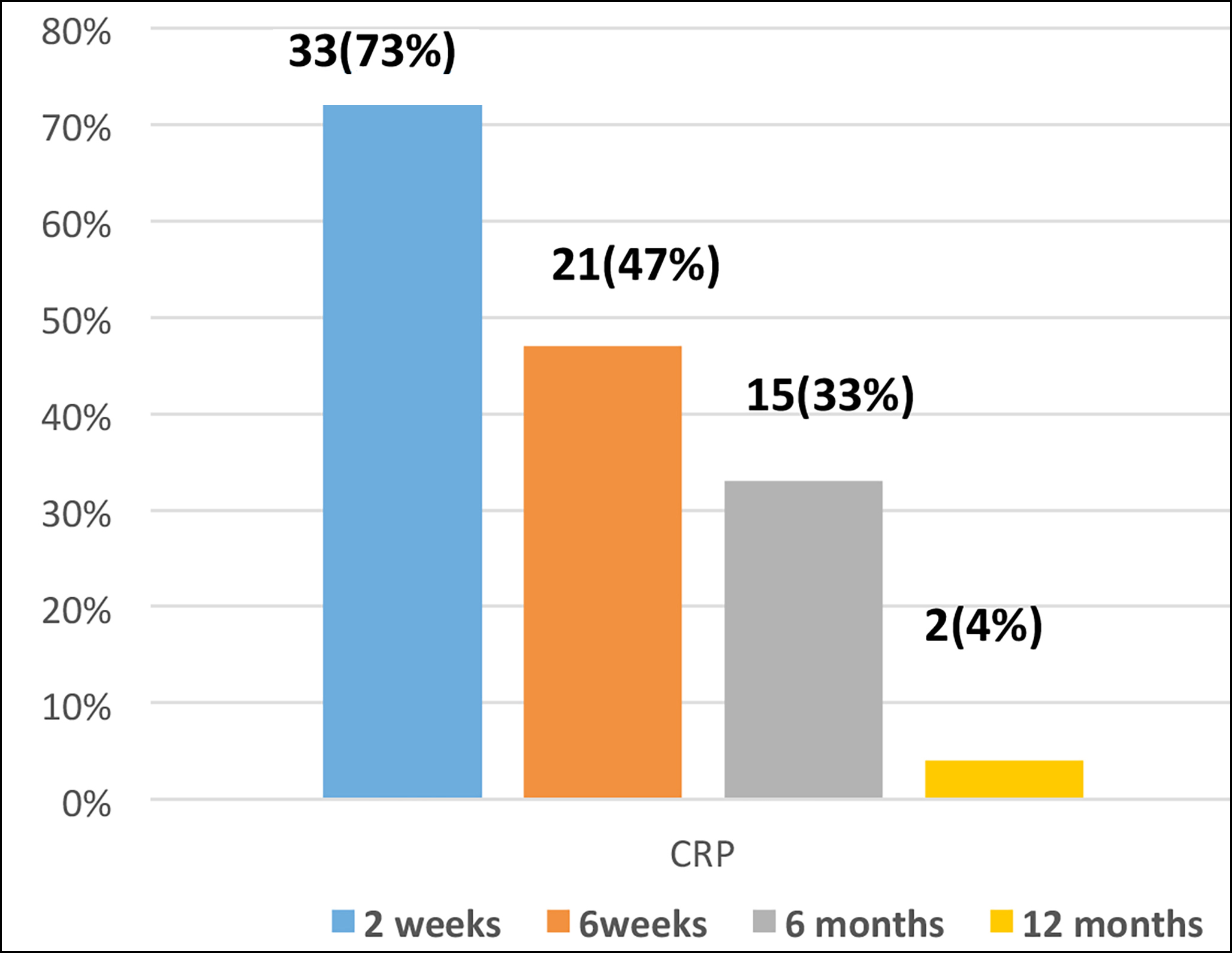 Figure 1: Decline pattern of C-reactive protein (CRP) over 12 months period.
Figure 1: Decline pattern of C-reactive protein (CRP) over 12 months period.
DISCUSSION
COM is difficult to treat bone infection, despite advancements in modern operative techniques the associated morbidity is very high probably due to difficulty in eradicating the infection.13,14 OM is a condition that commonly affects elderly patients, typically as a result of an infection spreading from nearby infected soft tissue or joints, this is often observed in cases of diabetic foot wounds and chronic soft tissue infections. This finding is consistent with the results of this study, where we examined a specific elderly population was examined. In this analysis, 33% of the elderly participants had a history of diabetic foot infection, further supporting the previously reported connection between OM and these types of infections in older individuals.15
Cases of COM that occur after trauma or post-surgical procedures are classified as non-hematogenous osteomyelitis. This type of osteomyelitis is more commonly observed in younger individuals.16 In this study, a marker proportion of adults (60.7%) with COM had a history of trauma and non-united unhealed fractures. This suggests that the development of COM in these cases may be directly linked to the traumatic event or surgical procedure. The presence of non-united unhealed fractures indicates that the bone healing process was interrupted or compromised in some way. This can create an environment that is more susceptible to infection, potentially leading to the development of osteomyelitis.14,15 Overall, this study supports the association between trauma, non-united unhealed fractures, and the development of non-hematogenous osteomyelitis in adults.
In cases of non-hematogenous OM, the infection is typically caused by a single organism, although there have been reports of polymicrobial cases as well.17 In this study, almost all cases of OM were associated with the isolation of a single organism. According to the available literature, the most common pathogen associated with OM is Staphylococcus aureus. In fact, one study reported a prevalence of S. aureus of approximately 52%, with 18% of those cases being MRSA; in the present study, the majority of OM cases were caused by gram-positive bacteria, particularly S. aureus. It is worth noting that a marked proportion (31%) of these cases were attributed to MRSA findings consistent with other studies.6,10 One possible explanation for this high incidence of MRSA is the prolonged use of antibiotics due to a history of non-healing ulcers, which can lead to the emergence of antibiotic-resistant strains, as already mentioned by different authors in literature.7
Bone infections are commonly treated with a prolonged course of antibiotics, typically lasting at least 6-8 weeks. However, this approach can result in selective pressure, killing susceptible bacteria while allowing antibiotic-resistant bacteria to survive and multiply, ultimately leading to treatment failure.18,19 To address this issue of bacterial persistence and treatment failure, the implementation of antimicrobial stewardship programs is necessary to ensure appropriate and judicious use of antibiotics.20
This study aimed to introduce antibiotic strategies that were previously mentioned in the literature, specifically targeting patients who had already undergone multiple courses of antibiotics. The authors employed a pre-defined empirical therapy based on the most recent antibiogram, followed by culture-based specific treatment with a de-escalation strategy based on the patient's clinical condition. Through the judicious use of antibiotics, the majority of the patients exhibited a positive response, with a recovery rate of 95.5%, highlighting the significance of antibiotic stewardship. The use of empirical therapy, guided by the hospital antibiogram, also helped rationalise the use of antibiotics and reduced the emergence of resistant strains. Numerous recent studies have placed extensive emphasis on formulating antibiograms and implementing empirical therapy as part of antibiotic stewardship in healthcare settings.11,20 This approach has gained considerable relevance and acceptance in the field of medicine, as it can significantly diminish the emergence of drug resistance while simultaneously improving clinical outcomes.
In this study, the authors implemented the use of non-conventional drugs such as doxycycline, Cotrimoxazole, and Rifampicin as oral options for treating cases of COM. These antibiotics have already been shown to produce excellent results and have been mentioned in the literature as effective treatments for chronic osteomyelitis.9 The majority of the present cases showed a good response when treated with a combination of surgical intervention and antibiotic therapy, which was monitored through the use of inflammatory markers and clinical assessments. The use of these non-conventional medicines not only led to good patient compliance but also contributed to a decrease in hospital stay. It is worth noting that these findings are consistent with existing literature that supports the efficacy of these drugs in the treatment of chronic osteomyelitis.15
However, a small percentage (4.4%) of the patients failed to respond even after receiving multiple courses of antibiotics and surgical debridement. This outcome aligns with findings from other studies that suggest a success rate for osteomyelitis ranging from 60 to 90%.17
The main limitation of this study was the small sample size and observational type of study as there should be a randomised controlled trial to compare the groups to look for better efficacy of antimicrobials.
CONCLUSION
COM is a complex clinical entity which is difficult to manage because of the increasing prevalence of resistant strains seen in this study. Antimicrobial resistance represents a daunting challenge with limited treatment options, however, this can be overcome by effective stewardship programs with accurate diagnostic reporting and judicious use of antimicrobials.
ETHICAL APPROVAL:
An approval has been taken prior to the study from the Institutional research and ethical committee of CMH Malir Cantt, Karachi, Pakistan (file no: 24/2022/Trg/Ere).
PATIENTS’ CONSENT:
Informed consent has been taken from all patients enrolled in this study regarding publication of the data keeping the confidentiality of every patient.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
AA: Design, data collection and its acquisition, write-up, and critical appraisal of the work.
AM: Conception and design of the work and data collection.
IAM: Conception and revising it critically for important intellectual content.
FS: Acquisition, analysis and interpretation of the data, literature review, and editing.
SRAN: Revising it critically for important intellectual content, final approval of the version to be published.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Jerzy K, Francis H. Chronic osteomyelitis-bacterial flora, antibiotic sensitivity and treatment challenges. Open J Orthop 2018; 12:153-63. doi: 10.2174/1874325001812010153.
- Gimza BD, Cassat JE. Mechanisms of antibiotic failure during Staphylococcus aureus osteomyelitis. Front Immunol 2021; 12:638085. doi:10.3389/fimmu.2021.638085.
- Fantoni M, Taccari F, Giovannenze F. Systemic antibiotic treatment of chronic osteomyelitis in adults. Eur Rev Med Pharmacol Sci 2019; 23(2 Suppl):258-70. doi: 10.26355/ eurrev_201904_17500.
- Gornitzky AL, Kim AE, O’Donnell JM, Swarup I. Diagnosis and management of osteomyelitis in children: A critical analysis review. JBJS reviews 2020; 8(6):e19. doi: 10.2106/ JBJS.RVW.19.00202.
- Gupta R, Gupta AK, Gupta S. Antibiotic sensitivity pattern of bacterial isolates in chronic osteomyelitis in a tertiary care teaching hospital of North India. J Pharm Negat Results 2022:3716-9. doi:10.47750/pnr.2022.13.S08.459.
- Khalid, Ihsanullah, Inam M, Shabir M. Frequency of different bacteria and their antibiotics sensitivity pattern in chronic osteomyelitis. J Pak Orthop Assoc 2020; 32(02):92–6. Retrieved from https://mail.jpoa.org.pk/index.php/upload/ article/view/429
- Fily F, Ronat JB, Malou N, Kanapathipillai R, Seguin C, Hussein N, et al. Post-traumatic osteomyelitis in Middle East war-wounded civilians: Resistance to first-line antibiotics in selected bacteria over the decade 2006–2016. BMC Infect Dis 2019; 19(1):1-8. doi: 10.1186/S12879-019-3741-9.
- Shenoy PA, Vishwanath S, Bhat SN, Mukhopadhyay C, Chawla K. Microbiological profile of chronic osteomyelitis with special reference to anaerobic osteomyelitis in a tertiary care hospital of coastal Karnataka. Tropical Doctor 2020; 50(3):198-202. doi: 10.1177/0049475520921283.
- Cortes-Penfield NW, Kulkarni PA. The history of antibiotic treatment of osteomyelitis. Open Forum Infect Dis 2019; 6(5):ofz181. doi: 10.1093/OFID/OFZ181.
- Kavanagh N, Ryan EJ, Widaa A, Sexton G, Fennell J, O'Rourke S, et al. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin Microbiol Rev 2018; 31(2):e00084-17. doi: 10.1128/ CMR.00084-17.
- Ferreira N, Reddy K, Venter RG, Centner CM, Laubscher M. Antibiogram profiles and efficacy of antibiotic regimens of bacterial isolates from chronic osteomyelitis of the appendicular skeleton: A developing-world perspective. S Afr Med J 2021; 111(7):642. doi: 10.7196/SAMJ.2021.V111I7.15516.
- Besal R, Adamic P, Beovic B, Papst L. Systemic antimicrobial treatment of chronic osteomyelitis in adults: A narrative review. Antibiotics (Basel) 2023; 12(6):944. doi: 10. 3390/antibiotics12060944.
- Clinical Laboratory Standards Institute. M100: Performance standards for antimicrobial susceptibility testing: 31st informational supplement; 2021.
- Wong D, Holtom P, Spellberg B. Osteomyelitis complicating sacral pressure ulcers: Whether or not to treat with antibiotic therapy. Clin Infect Dis 2019; 68(2):338-42. doi: 10.1093/cid/ciy559.
- Andrianasolo J, Ferry T, Boucher F, Chateau J, Shipkov H, Daoud F, et al. Pressure ulcer-related pelvic osteomyelitis: Evaluation of a two-stage surgical strategy (debridement, negative pressure therapy and flap coverage) with prolonged antimicrobial therapy. BMC infect dis 2018; 18(1):1. doi: 10. 1186/S12879-018-3076-Y.
- Zhang X, Yang X, Chen Y, Wang G, Ding P, Zhao Z, et al. Clinical study on orthopedic treatment of chronic osteo-myelitis with soft tissue defect in adults. Int Wound J 2022; 19(6):1349-56. doi: 10.1111/IWJ.13729.
- Barakat A, Schilling WH, Sharma S, Guryel E, Freeman R. Chronic osteomyelitis: A review on current concepts and trends in treatment. Orthop Trauma 2019; 33(3):181-7. doi: 10.1016/j.mporth.2019.03.005.
- Sananta P, Huwae TE, Ronadi D, Siahaan LD. The antibiotic use in osteomyelitis infection: A systematic review. Open Access Maced J Med Sci 2021; 9(F):720-3. doi: /10.3889/ oamjms.2021.7680.
- Young MJ, Hall LML, Merabishvilli M, Pirnay JP, Clark JR, Jones JD. Phage therapy for diabetic foot infection: A case series. Clin Ther 2023; 45(8):797-801. doi: 10.1016/j.clinthera.2023.06.009.
- Masetla MA, Ntuli PN, Abraham V, Godman B, Witika BA, Mudenda S, et al. Antimicrobial stewardship for outpatients with chronic bone and joint infections in the orthopaedic clinic of an academic tertiary hospital, South Africa. Antibiotics (Basel) 2023; 12(7):1142. doi: 10.3390/antibiotics12071142.